
Though we define the Fire as a rapid, self sustaining oxidation process accompanied by the evolution of heat, smoke & light of varying intensities, but scientifically, it is a very complicated phenomenon. Depending upon the physical conditions of atmosphere, type & distribution of fuels & oxidizing agents involved, the kinetic & chemistry of fire changes. The hot, toxic and dangerous gases as shown in fig.-1, are generated in the form of smoke in the upper zone of the fire in general, carbon monoxide, water vapours and unburnt carbon particles form the main constituents of smoke but in particular when plastics, specially polyurethane foams are involved in fire, apart from the above constituents, hydrogen cyanide and hydrogen chloride gases, are also evolved. If a hydrogen chloride gases are also evolved. If a person is exposed to lethal concentrations of carbon monoxide and hydrogen cyanide, the chances of his survival are almost negligible. This is the reason that the use of polyurethane foam for upholstery and decorating purposes is decorating purposes is discouraged. It is universally known that carbon monoxide(CO) is more dangerous than carbon dioxide(CO2) and damages caused to the persons exposed to CO are of permanent nature. The function of oxygen(O2) of blood purification is completely negated by CO when it enters in the human body through respiratory system & reacts with the blood.
ROLE OF OXYGEN IN HUMAN BODY
On breathing in a normal and unpolluted atmosphere air is filled in the lungs of a person. The oxygen from air joins with the Hemoglobin (Hb) of the blood and forms HbO.
![]()
This oxidized hemoglobin (HbO) flows with the blood to all parts and cells of the body. The blood acts as a carrier of oxygen to all the cells of the body. Further HbO on reaching the cell of the body, reacts with them and oxidizes them.
![]()
Thus, Hb becomes free and flowing with the blood through heart again reaches in the lungs and repeats the process. All the tissues and muscles of each part of the body get oxidized and generate energy in this process. Infect oxygen itself can be called as energy needed by the body.
Under all situations (Sleeping or awakened) the heart and brain of a person function continuously after getting energy (O2) through blood circulation. In case blood does not reach the brain or heart, they will become idle.
The process of digestion of food, which we eat, is a very complicated one. Many types of enzymes are produced by our body. These enzymes are made of proteins and function as catalyst. It is a bio chemical process. By the digestion of food, glucose is formed and this glucose reaches to all the cells continuously in our body.
Glucose, which is produced by the digestion of food in the body, reacts with the oxidized cells and provides energy to the body
![]()
In this way, all parts and muscles to the body get energy become fresh active and capable to do work. When we take deep and long breaths in pure air and inhale more air, more oxygen goes in our lungs and we feel more energetic and active. On exhalation more CO2. This is the way, how a person gets more energy through ‘Pranayam’. In pranayam, we inhale more air as per capacity of our lungs and practice and deep the same inside the lungs as long as possible. This increase the capacity of our lungs and as long as possible. This increase the capacity of our lungs and make them strong. Thus inhaled oxygen gets more time to stay inside the lungs to provide more techniques by which our Rishimunihis, Yogies and munis live. Healthy life.
From the above it is clear that our brain remains active, heart functions perfectly and continuously and we get unlimited energy only when we breath in pure air.The constituents of pure air which has been gifted to use by nature are Nitrogen -78 , Oxygen -21 , CO2 and other inert gases -1/. Obviously, the presensnce of extra and additional CO2 will spoil the natural balance of air and ultimately will be extremely dangerous if we breath in it. It is a chance of survival, if a person is exposed to CO2, but if a person inhale CO beyond a particular limit, the survivability is least
IMPORTANT FACTS ABOUT CARBON MONOXIDE(CO)
State : Carbon monoxide is a colour less and smell less gas due to which it becomes difficult to identify and feel it. In other words, we can say that CO is an invisible and tasteless killing gas. It is a natural oxide, slightly soluble in water and highly poisons.
Combustibility: It burns with a blue flame under following flammability limits
Lower Flammability limit = 12/5 .. (v/v) air
Upper Flammability Limit = 74/2.. (v/v) air
Auto-ignition temperature =651.1C
Under controlled burning condition, the carbon of most organic materials can be oxidized completely by supplying excess oxygen. In the uncontrolled burning of an accidental fire, the availability of oxygen is never ideal and some of the carbon is incompletely oxidized to carbon monoxide. In a confined smoldering fire, the ration of carbon monoxide (CO) to Carbon dioxide (CO2) is usually greater than in well ventilled free burning fire. It is abundantly present under all fire conditions and therefore is the major threat in most fire atmosphere.
POISONOUS:-
When a person stays in an atmosphere of 0.1/ CO air (v/v) for 30 minutes ,25/ of total hemoglobin of his blood is rendered incapable of functioning.
When a person breath in 0.32/ CO in air(v/v) for 30minutes , he will face death.
When a person takes 3-4 breaths in an atmosphere of 1.3/ CO (v/v), he will become senseless.
When a person breaths in pure CO, instantaneously, he will becom senseless and may face death in few minutes.
WHY CARBON MONOXIDE SPREADS SO FAST IN AIR?
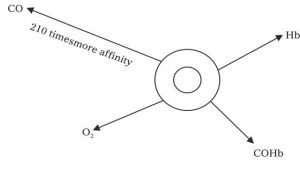
The vapour density of air is 14.4 and vapour density of CO is 14.0. Obviously, the vapour densities of air and CO both are approximately same so CO mixes with air instantaneously and spread very fast in air.
HOW CARBON MONOXIDE CAPTURES HAEMOGLOBIN MECHNISM
When a person breath in a polluted atmosphere containing carbon monoxide, the CO gas goes inside along with other gases i.e. oxygen, nitrogen, oxygen, etc. in the lungs. Carbon monoxide has 210 times faster than oxygen and forms stable compound after reacting with the Hb of blood
![]()
Carboxylhaemoglobin is a very stable compound and forms a very strong layer around Hb and leave no chances for Hb and Oxygen to form HbO and reach to the cells, tissues and other parts of the body.
In our body, any natural mechanism which can act to get rid of CoHb,does not exist and slowly slowly all the molecules oh Hb get arrested by Co to form CoHb. At this stage , Hb is totally incapable to carry oxygen to different parts of the body like brain, heart etc. in the initial stages, due to non-availability of oxygen, the natural functioning of the brain gets retarded and the brain becomes idle. As more CO enter in the body, the body is further deprived of oxygen then chocking of the breath starts , man gets deeply fainted and finally dies. The persons who have consumed alcohol or used some intoxicants are fastly effected by CO.
AREAS OF DANGERS
The plastics like PVC, polyurethane foam Acrylics etc have found an unavoidable place in our society and they are used for upholstery and decoration purposes and also as main building construction materials. Under fire conditions the CO and HCN produced from the above materials travel from one part of the building to the other part in no time and lays a death trap.
Unlimited number of industries has come up in the name of so-called development but has caused maximum damage to the atmosphere and mankind. CO and CO2 are produced in the form of dreadful smoke from industries.
Copper oxide is reduced in presence of CO to form cooper and dioxide.
![]()
In this reaction, the presence of highly combustible and very poisonous carbon monoxide gas in a reducing atmosphere poses an explosion thereat to the fire fighter, if ventilation of the space suddenly occurs. The breathing in such atmospheres without self contained breathing apparatus is highly dangerous.
Though a very remote situation, but sometimes it can happen that during fire fighting operation water is applied on carbon. If the control of fire takes to long time then carbon and water(steam) under high temperature conditions produce ”water Gas”
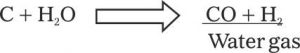
This reaction can take place at any time during an intensely hot fire. Both the hydrogen gas and carbon monoxide are Flammable reducing agents and can cause danger to the fire fighters or occupants.
On 14.05.2015 shri Ashok Pawar along with other labor workers work in well redevolment in forest area for increasing depth of water level in rainy season on diesel generator pump bt the depth was at about 35ft deep from ground level, there by increased the concentration of carbon monoxide and as a result, 35 yrs old labor namely Mr.sudhir pawar and Mr.Ashok Pawar 40yrs old found dead and other 02 labor severely injured due to suffocation of carbon particles.
PREPARATION :-
It is formed by incomplete combustion of carbon ad carbon containing fuels.
![]()
This type of incomplete combustion occurs during burning of petrol or diesel in automobiles and therefore, carbon monoxide is always present in automobile exhausts.
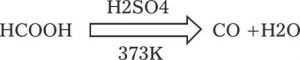
It is also prepared in the laboratory by dehydration of formic acid with conc. H2SO4 at 373K
The buses, cars, trucks, motor cycles and scooters produce CO when run on the road.
Burning of wood under condition of an insufficient supply of oxygen produces copious amounts of CO so se of B.A> Set is essential while doing fire fighting under such situations.
SYMPTOMS-WHEN A PERSON IS AFFECTED BY CARBON MONOXIDE
- Several headache
- Dizziness
- Idleness of mind
- Chest congestion
- Weakness in arms and legs
- Vomiting
- Asphyxia
- Death
WHAT SHOULD BE DONE
- Do not be panic
- Do not have fear
- Take the victim immediately in fresh air
- Remove the shoes and tight cloths of the victims
- Keep the victim warm
- Do not crowed around the victim
- Turn the mouth in onr direction, bring the tongue out and keep the mouth open
- Give artificial breathing respiration till the first aid and medical aid is provided
- Do not provide anything to eat or drink to the victim.
- Check the level of CO in the suspected area
- If the conc. Of CO is found more than the safe limits, cordon off the area and nobody should be allowed to remain in the area
FOR FIRE FIGHTERS
Water spray sometimes may not be that much effective in the CO filled atmosphere so apply the extinguish ant carefully and intelligently
Enter into a highrise building for fire fighting, fully equipped with B.A.Set and Other safety devices.
WHICH CAUSES MORE WORRY TO FIRE FIGHTER (CO OR CO2)
Clearly CO causes more worry than CO2 because CO cause permanent damages of the body. Some physical properties of both are give as :
|
Property |
CO |
CO2 |
|
Melting Point(K) |
68 |
216.4 at 5.2 |
|
Boiling Point(K) |
81.4 |
94.5(subline) |
|
Density (gL) at 273K |
1.250 |
1.977 |
|
Heat of formation (kJ Mol) |
-110.5 |
-393.5 |
CONCLUSION:-
The damages caused by carbon monoxide are mostly irreversible; hence extra care should always be taken under all circumstance. Use of polyurethane foam, for upholstery and decoration purposes should be totally stooped otherwise also the use of all plastics must be minimized. If somebody has become the victim of CO then immediately, he should be given artificial breathing and taken to the nearest hospital for treatment, victim should never be left alone, somebody should always accompany him. All the vehicles should be checked for pollution cases y CO and the pollution level should always be kept below dangerous levels. The fire fighters should take all precautionary measures while fighting fire under such situations.
References ;
- Principles of Fire protection Chemistry by Tune.
- Fire Protection Hand book NFPA
- Research and Development work done at defense institute of fire Research




















































